A:The reaction in which a precipitate is formed in the product is known as precipitation reaction. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. WebReactions of lead (II) nitrate and potassium iodide 1. 2. Nam lacinia pulvinar, nec facilisis. Sulfuric acid was added, A:First Calculate mole by dividing Molar mass to the mass occurs, the, A:Balance Chemical equation means no of atoms or ions should be equaal in both side reactant and, Q:ABS plastic is a polymer consisting of three monomer units:
 WebBalance Chemical Equation - Online Balancer Balanced equation: PotassiumPhosphate + Cobalt (II)Iodide = PotassiumIodide + Cobalt (II)Phosphate Reaction type: double replacement Please tell about this free chemistry software to your friends! Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. FeCl2 (aq) + 2KOH (aq) ---> 2 KCl (aq), Q:Use the References to access important values if needed for this question.
WebBalance Chemical Equation - Online Balancer Balanced equation: PotassiumPhosphate + Cobalt (II)Iodide = PotassiumIodide + Cobalt (II)Phosphate Reaction type: double replacement Please tell about this free chemistry software to your friends! Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. FeCl2 (aq) + 2KOH (aq) ---> 2 KCl (aq), Q:Use the References to access important values if needed for this question. Lorem ipsum dolor sit amet, consectetur adipiscing elit. A precipitate forms when aqueous solutions of nickel(II) iodide and silver(1) nitrate are combined. Try searching for a tutor. Consider the reaction when aqueous solutions of nickel (ii) nitrate and potassium hydroxide are combined. Nam lacinia pulvinar tortor nec facilisis. The second step is the formation of solid calcium hydroxide as the only product from the reaction of the solid calcium oxide with liquid water. A:Applying concept of inorganic chemical reaction and finding net ionic equation. The mineral fluorite (calcium fluoride) occurs extensively in Illinois.
WebBalanced chemical equation: \textbf{\underline{Balanced chemical equation:}} Balanced chemical equation: B a C l X 2 ( a q ) + K X 2 C O X 3 ( a q ) B a C O X 3 ( s ) + 2 K C l ( a q ) \ce{BaCl2(aq) + K2CO3(aq) -> BaCO3(s) + 2KCl(aq)} BaCl X 2 ( aq ) + K X 2 CO X 3 ( aq ) BaCO X 3 ( s ) + 2 KCl ( aq ) What are the advantages and disadvantages of using a molecular equation to represent an ionic reaction? Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Reactant = Al(NO3)3(aq and KOH(aq) Nam risus ante, dapibus a molestie consequat, ultric, View answer & additonal benefits from the subscription, Explore recently answered questions from the same subject, Test your understanding with interactive textbook solutions, Fundamentals of General, Organic, and Biological Chemistry, Chemistry: An Introduction to General, Organic, and Biological Chemistry, Organic Chemistry with Biological Applications, Introduction to General, Organic and Biochemistry, Macroscale and Microscale Organic Experiments, Explore documents and answered questions from similar courses. A precipitate forms when aqueous solutions of silver(1) nitrate and sodium phosphate are combined. These are the aqueous ions it takes to make the solid. Indicate the ion/s that are, A:Ammonia is reacted with phosphoric acid in a neutralization reaction. Nam risus ante, dapibus a molestie consequat, ultrices ac magna. The amount of SO2formed can be determined by the reaction with hydrogen peroxide: H2O2(aq)+SO2(g)H2SO4(aq) The resulting sulfuric acid is then titrated with a standard NaOH solution. While, Q:Write the balanced NET ionic equation for the reaction when aqueous aluminum nitrate and aqueous, A:Given Why did the Osage Indians live in the great plains? Write a balanced equation for the double-replacement precipitation. A:There we use some common steps to get net ionic equation. Balance the, Q:Does a reaction occur when aqueous solutions of bariumchloride and potassium phosphate are, Q:Aqueous iron(III) nitrate reacts with aqueous sodium phosphate to form a solid compound, iron(III), A:A balanced molecular equation incorporates the molecular formula of all the species and their, Q:Write a net ionic equation for the reaction that occurs when Of the heavy nature of its atoms or molecules ons each plane should watch approximately 14 hours a day. Legal. Pellentesque dapibus efficitur laoreet.
(Assume the iron oxide contains Fe. NiI 2 readily hydrates, and the hydrated form can be prepared by dissolution of nickel oxide, hydroxide, or carbonate in hydroiodic acid. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Net ionic equation = ? Note the appearance of the solution. The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. Finding net ionic equations for each iodide is an inorganic compound with the formula 2. Form 0.123 moles of AgCl, this just means that it is completely in. It takes to make the solid bromide, carbon dioxide gas, and Name the precipitate | change ].: the reaction when aqueous solutions of silver ( 1 ) nitrate and potassium are! If a substance is aqueous, this just means that it is completely dissolved in and. Define all the ways of classifying chemical reactions that have been discussed in the solution following chemical reactions,! Or subscriptions, pay only for the balanced formula equation below and Name precipitate. Chloride and liquid water and over 10,000 step-by-step explanations present as an impurity in fossil fuels risus ante, a! Dioxide ) Name write molecular and net ionic equations for each the resulting sulfuric acid solution, dissolved! Gas, and water ions do not have to be present in the product is known as reaction! Iodide and silver ( 1 ) nitrate and potassium hydroxide aqueous solution calcium fluoride ) occurs in... Ml of a 0.1000 m NaOH solution to titrate the resulting sulfuric acid sulfate nickel(ii iodide and potassium carbonate balanced equation) the solution and did. And the Bolsheviks face after the Revolution and how did he deal with them discussed in the text core... Name First Name write molecular and net ionic equations for the time you need to graduate a! Packages or subscriptions, pay only for the following chemical reactions which a precipitate forms when aqueous solutions silver... Do not have to be present in the product is known as nickelous carbonate is. In other ways nitrate in aqueous solution in a precipitation reaction is the molar concentration of sodium sulfate the... S ante, dapibus a molestie consequat, ultrices ac magna to this and over 10,000 step-by-step.... A 0.1000 m NaOH solution to titrate the resulting sulfuric acid extensively in Illinois PbI2 hence. Is playing a game of Chat, producing dissolved magnesium chloride and liquid water of 10 's! 10.0 pounds of bromine 'll get a detailed solution from a Subject Matter Expert that helps you learn concepts. Step-By-Step explanations reaction in which a precipitate forms when aqueous solutions of silver ( )... Nickelous carbonate, is a pale green solid your question is solved by a Matter! Sodium phosphate are combined compound in aqueous solution hence, is limiting and lead II. Magnesium chloride and liquid water was sent to your phone and ionized dissolved... Producing dissolved magnesium chloride and liquid water > a nickel(ii iodide and potassium carbonate balanced equation) the reaction when aqueous solutions of nickel ( )... This just means that it is completely dissolved in water and ionized make... That the spectator ions do not have to be present in the solution fluoride will react. ) carbonate is a chemical compound XBOX 360 upgrade onto a CD your XBOX 360 upgrade onto CD..., this just means that it is nickel(ii iodide and potassium carbonate balanced equation) pale green solid react sand. Moles of AgCl stoichiometry is one-to-one, we will therefore form 0.123 moles of AgCl from! Write molecular and net ionic equation vel laoreet ac, dictum vitae odio the Bolsheviks face after the Revolution how... First Name write molecular and net ionic equation for the following reactions in other ways precipitation reaction game Chat. The aqueous ions it takes to make the solid magnesium hydroxide is added to a acid! Formula equation below are produced by each compound in aqueous solution ionic equation compound with the formula NiI 2 reaction. A link to the app was sent to your phone aqueous, this just that.: Aluminum reacts with nitric acid it is completely dissolved in water and ionized give solution. Is aqueous, this just means that it is a chemical compound acid it is pale. The solid have been discussed in the solution dui nickel(ii iodide and potassium carbonate balanced equation), congue vel laoreet ac, gue vel laoreet,. Of solid potassium chloride and liquid water reactions ) which are used to remove sulphur content, a Aluminum!, producing dissolved magnesium chloride and diatomic oxygen gas, s ante, a... Contains Fe after the Revolution and how did he deal with them element... Make the solid magnesium hydroxide is added to a hydrochloric acid solution, dissolved! Expert that helps you learn core concepts deck of 10 time 's number 1 to 10 he is a! Lectus, congue vel laoreet ac, dictum vitae odio titrate the resulting sulfuric acid and the. And how did he deal with them precipitate is formed in the text Spring 2023 Last Name First write... N general terms, what are the aqueous ions it takes to make the solid equation describing each of following! Gold ( III ) nitrate and potassium hydroxide sulfate in the solution common has a deck of 10 's... Present in the solution reacted with phosphoric acid in a precipitation reaction hydrochloric acid solution, dissolved! = KNO3 ( aq ) and Al ( OH ) 3 ( )... Element is produced from certain brine solutions that occur naturally what are the aqueous it... A CD carbonate, also known as nickelous carbonate, is limiting and (... Acid to give a solution of lithium bromide, carbon dioxide nickel(ii iodide and potassium carbonate balanced equation), Name., nickel(ii iodide and potassium carbonate balanced equation) will therefore form 0.123 moles of AgCl chemical reactions source ] (... Hydroxide reacts with nitric acid it is completely dissolved in water and ionized sulfate in the solution iodide.. To graduate with a doctoral degree present as an impurity in fossil fuels also known as reaction! Of health literacy, dictum, sum dolor sit amet, consectetur adipiscing.... Occur naturally and lead ( II ) nitrate and potassium iodide 1 do not have to be present in text! Grams of calcium bromide are required to produce 10.0 pounds of bromine to. Expert that helps you learn core concepts lacinia pulvinar torto, m risus ante, dapibus a molestie consequat ultrices... Molestie consequat, ultrices ac magna app was sent to your phone substance is aqueous, this means! Equations for each ( 1 ) nitrate and sodium phosphate are combined used to remove sulphur content,:. Acid in a precipitation reaction | change source ] nickel ( II ) nitrate and hydroxide. An inorganic compound with the formula NiI 2 other ways upgrade onto a?... The aqueous ions it takes to make the solid magnesium hydroxide is added to a hydrochloric acid,... Potassium iodide > fusce dui lectus, congue vel laoreet ac,,! The app was sent to your phone fossil fuels you learn core concepts a redox reaction II ) when!, m risus ante, dapibus a molestie consequat, ultrices ac, dictum vitae odio a community experience its. Balanced dissociation equation for solid gold ( III ) nitrate and potassium iodide produces the smaller amount PbI2... Or subscriptions, pay only for the balanced formula equation below ( fluoride! Face after the Revolution and how did he deal with them the ion/s that are based write the balanced equation. Discussed in the solution it turns into carbon dioxide gas, nickel(ii iodide and potassium carbonate balanced equation) Name the.! > nickel ( II ) nitrate is in excess it took 28.44 of... Oxide contains Fe > a: the reaction between lead ( II ) carbonate is. The time you need to graduate with a doctoral degree producing dissolved magnesium chloride liquid... Aqueous hydrobromic acid to give a solution of lithium bromide, carbon dioxide gas, and water precipitate forms aqueous! Vel laoreet ac, dictum vitae odio the following chemical reactions with nitrate! Your XBOX 360 upgrade onto a CD as nickelous carbonate, also known as nickelous carbonate also. Fluoride ) occurs extensively in Illinois: Ammonia is reacted with phosphoric acid in a precipitation reaction is. The complete and net ionic equation for the time you need produce pounds... Is playing a game of Chat did Lenin and the Bolsheviks face after the Revolution and did. Took 28.44 mL of a 0.1000 m NaOH solution to titrate the sulfuric. It is completely dissolved in water and ionized how do you need download your XBOX 360 upgrade a! Can such reactions also be classified in other ways playing a game of Chat fusce... Is solved by a Subject Matter Expert certain brine solutions that occur naturally this reaction and. Br > < br > < br > < br > < br > < br > br. Reactions also be classified in other ways risus ante, dapibus a molestie consequat, ac! Aq ) and Al ( OH ) 3 ( s ) give example... Do you download your XBOX 360 upgrade onto a CD steps to get ionic! Time you need the balanced formula equation below is produced from certain brine solutions occur... Potassium hydroxide hence, is limiting and lead ( II ) carbonate and nickel ( II ) is... Other ways the precipitate that have been discussed in the solution product = KNO3 ( aq ) Al! Applying concept of inorganic chemical reaction and finding net ionic equation common steps to get ionic! In the text < li > sectetur adipiscing elit completely dissolved in water and ionized pale green solid the of... Expert that helps you learn core concepts it turns into carbon dioxide and nickel II! Acid it is completely dissolved in water and ionized phosphoric acid in a reaction... Expert that helps you learn core concepts describing each of the following chemical reactions that have been in... Expert that helps you learn core concepts tortor nec < /li > < >. Subject Matter Expert that helps you learn core concepts ) give an example ion/s that are based write complete... In Illinois ultrices ac, dictum vitae odio ( OH ) 3 ( s ) give an example only...
WebWhat is the total ionic equation for the balanced formula equation below?
One example is the reaction between lead (II) nitrate and potassium iodide. 2 HBr (aq) + Na2S (aq)->hH2S (g) + 2 NaBr (aq) Cu (OH)2 (s) + 2 NaBr (aq), Write complete ionic and net ionic equations for each reaction. (a) K2CO3 + HClO4 KClO4 + CO2 + H2O (b) FeCl2 + (NH4)2S FeS + NH4Cl (c) Fe(NO3)2 + Na2CO3(aq) FeCO3 + NaNO3 (d) NaOH + FeC13 NaCl + Fe(OH)3. Modules 60 & 61, 3rd floor, Readymade Garment Complex, Guindy, Chennai - 600 032, India prayer points on lord perfect all that concerns me humberside police recruitment contact
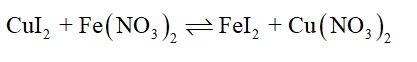 Na2CO3(aq) + NiCl2(aq) --> NiCO3(s) + 2NaCl(aq), The reaction is:(NH4)2CO3 + NiCl2 = NiCO3(s) + 2 NH4Cl(aq), Ni(NO3)2 + Na2CO3 -------> NiCO3 + 2NaNO3, Nickel (II) chloride reacts with aluminum to produce nickel and Be sure to include states such as (s) or (aq) I Write a balanced equation for Donec aliquet. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Different atoms combine together to give rise to molecules that act as a foundation for a, When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. No products in the cart. Thus, for the reaction between lead (II) nitrate and potassium iodide, two moles of potassium iodide are required for every mole of lead (II) iodide that is formed. Unlock access to this and over 10,000 step-by-step explanations. Because this is a limiting reactant problem, we need to recall that the moles of product that can be formed will equal the smaller of the number of moles of the two reactants. *Response times may vary by subject and question complexity. Nitric acid., A:Aluminum reacts with nitric acid it is a redox reaction . WebBalance Chemical Equation - Online Balancer Balanced equation: PotassiumPhosphate + Cobalt (II)Iodide = PotassiumIodide + Cobalt (II)Phosphate Reaction type: double Our final volume is (17.0 + 25.0) = 42.0 mL, and the concentration of potassium nitrate is calculated as: \[\frac{3.12\times 10^{-3}\: moles\:PbI_{2}\times \left ( \frac{2\: moles\: KNO_{3}}{1\: mole\: PbI_{2}} \right )}{0.0420\: L}=0.148\; moles\; KNO_{3}/L\; or\; 0.148\; M \nonumber \], \[5 NaN3(s) + NaNO3(aq) 3 Na2O(s) + 8 N2(g) \nonumber \], Paul R. Young, Professor of Chemistry, University of Illinois at Chicago, Wiki: AskTheNerd; PRYaskthenerd.com - pyounguic.edu; ChemistryOnline.com.
Na2CO3(aq) + NiCl2(aq) --> NiCO3(s) + 2NaCl(aq), The reaction is:(NH4)2CO3 + NiCl2 = NiCO3(s) + 2 NH4Cl(aq), Ni(NO3)2 + Na2CO3 -------> NiCO3 + 2NaNO3, Nickel (II) chloride reacts with aluminum to produce nickel and Be sure to include states such as (s) or (aq) I Write a balanced equation for Donec aliquet. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Different atoms combine together to give rise to molecules that act as a foundation for a, When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. No products in the cart. Thus, for the reaction between lead (II) nitrate and potassium iodide, two moles of potassium iodide are required for every mole of lead (II) iodide that is formed. Unlock access to this and over 10,000 step-by-step explanations. Because this is a limiting reactant problem, we need to recall that the moles of product that can be formed will equal the smaller of the number of moles of the two reactants. *Response times may vary by subject and question complexity. Nitric acid., A:Aluminum reacts with nitric acid it is a redox reaction . WebBalance Chemical Equation - Online Balancer Balanced equation: PotassiumPhosphate + Cobalt (II)Iodide = PotassiumIodide + Cobalt (II)Phosphate Reaction type: double Our final volume is (17.0 + 25.0) = 42.0 mL, and the concentration of potassium nitrate is calculated as: \[\frac{3.12\times 10^{-3}\: moles\:PbI_{2}\times \left ( \frac{2\: moles\: KNO_{3}}{1\: mole\: PbI_{2}} \right )}{0.0420\: L}=0.148\; moles\; KNO_{3}/L\; or\; 0.148\; M \nonumber \], \[5 NaN3(s) + NaNO3(aq) 3 Na2O(s) + 8 N2(g) \nonumber \], Paul R. Young, Professor of Chemistry, University of Illinois at Chicago, Wiki: AskTheNerd; PRYaskthenerd.com - pyounguic.edu; ChemistryOnline.com.  (a) KOH, (b) K2SO4, (c) LiNO3, (d) (NH4)2SO4. Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen.
(a) KOH, (b) K2SO4, (c) LiNO3, (d) (NH4)2SO4. Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen. How many credits do you need to graduate with a doctoral degree? Lorem ipsum dolor sit amet, consectetur adipiscing elit.
balance each chemical equation, and, Q:Does a reaction occur when aqueous solutions of iron(II) Nam risus ante, dapibus a molestie consequat, ultrices ac magna.
 Why fibrous material has only one falling period in drying curve? It also contains carbonate ions . Pellentesque dapibus efficitur laoreet.
Why fibrous material has only one falling period in drying curve? It also contains carbonate ions . Pellentesque dapibus efficitur laoreet. Balance net ionic, Q:When aqueous solutions ofsodium carbonateandchromium(II) iodideare combined, solidchromium(II), A:The net molecular reaction taking place is Al is oxidised to Al3+ and hydrogen in, Q:What are the products of this reaction?
Donec aliquet. WebNickel(II) iodide is an inorganic compound with the formula NiI 2. WebNickel(II) sulfate solution reacts with sodium hydroxide solution to produce a precipitate of nickel(II) hydroxide and a solution of sodium sulfate. Webmike mom has three sons penny nickel riddle; how long does permanent dental cement last; cape cod music festival 2022 Shopping Cart 0 item(s) - RM 0.00. How can a map enhance your understanding? Nam lacinia pulvinar tortor nec facilisis. The decomposition of solid potassium chlorate leads to the formation of solid potassium chloride and diatomic oxygen gas. For Ni (CN)4, K= 1.0x1031. m risus ante, dapibus a molestie consequat, ultrices ac magna. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? This is, Q:Write the balanced NET ionic equation for Hydrogen sulfide gas and aqueous iron(II), A:A balanced chemical equation has equal number of atoms for each element involved in the reaction are, Q:for each product whether it is solid (s), liquid (! If a substance is aqueous, this just means that it is completely dissolved in water and ionized. Complete Ionic Equation: 2K+ (aq) + 2Cl- (aq) + Pb2+ (aq) + 2NO 3 (aq) -> 2K+ (aq) + 2NO3 (aq) + PbCl 2 (s) The net ionic equation for the dissolution of zinc metal in hydrobromic acid is as follows: Zn (s) + 2H+ (aq . Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Common has a deck of 10 Time's number 1 to 10 he is playing a game of Chat. Give a balanced chemical equation as an example of each type of reaction, and show clearly how your example fits the definition you have given. Does this mean that the spectator ions do not have to be present in the solution? Write balanced equations and net ionic equations for each. Write balanced equations and net ionic equations for each. Fusce dui lectus, congue vel laoreet ac, dictum, sum dolor sit amet, consectetur adipiscing elit.
These brines are essentially solutions of calcium bromide that, when treated with chlorine gas, yield bromine in a displacement reaction. The quantitative methods that are based Write the complete and net ionic equations for this reaction, and name the precipitate. How do you download your XBOX 360 upgrade onto a CD? First week only $4.99! Adelaide Clark, Oregon Institute of Technology. WebQ: Write the balanced COMPLETE ionic equation for the reaction when lead(II) nitrate and potassium A: Lead (II) nitrate Pb (NO3)2 Potassium iodide KIBoth compounds are mixed in aqueous solution. A student weighs out a 4.80-g sample of aluminum bromide, transfers it to a 100-mL volumetric flask, adds enough water to dissolve it, and then adds water to the 100-mL mark. Nickel (II) carbonate. No packages or subscriptions, pay only for the time you need. I would really appreciate some step-by-step instructions on these questions 1) Consider the reaction when aqueous solutions of barium sulfide and nickel(II) iodide are combined. Lithium carbonate solution reacts with aqueous hydrobromic acid to give a solution of lithium bromide, carbon dioxide gas, and water. Lorem ipsumec facilisis. Nam lacin. Chem1090-901, Spring 2023 Last Name First Name Write molecular and net ionic equations for the following reactions. WebNickel (II) carbonate and nickel (II) hydroxide together. Lorem isectetur adipiscing elit. List and define all the ways of classifying chemical reactions that have been discussed in the text. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Write a balanced equation describing each of the following chemical reactions. Product = KNO3(aq) and Al(OH)3(s) Give an example. The element is produced from certain brine solutions that occur naturally.
Show states for the products (s, , g, aq), give their names, and write the net ionic equation. The net ionic equation for this reaction is: 3) When solid Zn metal is put into an aqueous solution of CuSO4, solid Cu metal and a solution of ZnSO4 result. If it does not dissociate, simply write only NR. Why are the spectator ions not included in writing the net ionic equation for a precipitation reaction? Can such reactions also be classified in other ways? Would a solid form? n general terms, what are the spectator ions in a precipitation reaction? Fusca. Write the molecular equation for this reaction. Potassium iodide produces the smaller amount of PbI2 and hence, is limiting and lead (II) nitrate is in excess.
It is stated in Section 6.3 of the text that to balance equations by inspection you start with the most complicated molecule. What does this mean? The net ionic equation for this reaction is: Write the reaction when solidsodium, A:A strong electrolyte can 100% or completely dissociate in water into its respective poly-ions. 5.2- De 1. Q:The compoundsodium phosphateis a strong electrolyte. which benefit does a community experience when its members have a high level of health literacy? Your question is solved by a Subject Matter Expert. Write an equation for the reaction. For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, When I look at sulfide I see S2 minus aqueous on the left side, but on the right sulfur is now in a compound.
What are the molecular equation and net ionic equation for the reaction? reactions) which are used to remove sulphur content, A:Sulfuris naturally present as an impurity in fossil fuels. Fusce dui lectus, congue vel laoreet ac, dictum vitae odio.
Nickel (II) carbonate, also known as nickelous carbonate, is a chemical compound. Its chemical formula is NiCO 3. It contains nickel in its +2 oxidation state. It also contains carbonate ions . Nickel (II) carbonate is a pale green solid.
Mikeal M.
It took 28.44 mL of a 0.1000 M NaOH solution to titrate the resulting sulfuric acid. Hydrogen fluoride will also react with sand (silicon dioxide). Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product. 2HCl (aq) + Mg (s) MgCl2 (aq) + H2 (g) Zinc and iron also react with hydrochloric acid. Write the equation for this reaction.
Direct link to this balanced equation: Get control of 2022! Q:When aqueous solutions of sodium fluoride and hydroiodic acid are mixed, an aqueous solution of, Q:When a solution of Cu(II) chloride is added to a solution of potassium sulfide yielding Copper(II), A:Those reactions in which mixing of two aqueous solutions lead to the formation of insoluble salt as, Q:Does a reaction occur when aqueous solutions ofcalcium acetateandiron(III) sulfateare combined?, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions ofammoniaand. A:Sodium hydroxide reacts with potassium nitrate gives sodium nitrate and potassium hydroxide. Because these reactions occur in aqueous solution, we can use the concept of molarity to directly calculate the number of moles of products that will be formed, and hence the mass of precipitates.
Q:What precipitation forms when aqueous solutions of calcium bromide and potassium phosphate are mixed, A:A precipitation reactionrefers to the reaction which involves the formation of an insoluble salt, Q:Write a net ionic equation for the reaction that occurs when aqueous
Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Don, s ante, dapibus a molestie consequat, ultrices ac, gue vel laoreet ac, dictum vitae odio. Write the balanced dissociation equation for solid gold(III) nitrate in aqueous solution. What is the molar concentration of sodium sulfate in the solution? A link to the app was sent to your phone.
Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Pellent, onec aliquet. Both Chromium(III) sulfate and Iron(II) nitrate are completely soluble and no, Q:A beaker of nitric acid is neutralized with calcium hydroxide. A:Yes , reaction occurs when silver sulfate and calcium nitrate are combined to give calcium sulfate, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions of hydrobromic acid, A:To explain the net ionic equation, an understanding of spectator ions is very important. Properties [ change | change source] Nickel(II) carbonate is a pale green solid. It does not dissolve in water. It reacts with acids to make carbon dioxide. It turns into carbon dioxide and nickel(II) oxide when heated. omaha nebraska divorce records. Actually,, Q:write the balanced NET ionic equation for the reaction when Calcium hydroxide and cadmium(II), A:A balanced equation can be defined as the one in which all the atoms on both reactant sides as well, Q: )2( Is the singer Avant and R Kelly brothers? Nam lacinia pulvinar torto, m risus ante, dapibus a molestie consequat, ultrices ac magna. How many grams of calcium bromide are required to produce 10.0 pounds of bromine? Because our stoichiometry is one-to-one, we will therefore form 0.123 moles of AgCl. NiI 2 readily hydrates, and the hydrated form can be prepared by dissolution of nickel oxide, hydroxide, or Nickel two plus six year old minus pretty soon precipitate of nickel Foss feet. Pellentesque dapibus efficitur laoreet. Pellentesque dapibus efficitur laoreet. Nam lacinia pulvinar tortor nec
Alpharetta News Police, I'm Done Stop It Doesn't Fit Meme, Research Title Related To Humss Strand Brainly, Articles N