made of naphthalene, so it's a very distinctive smell. Solid animal fat, in contrast, contains mainly saturated hydrocarbon chains, with no double bonds.
Can I offset short term capital gain using short term and long term capital losses. Now, try dissolving glucose in the water even though it has six carbons just like hexanol, it also has five hydrogen-bonding, hydrophilic hydroxyl groups in addition to a sixth oxygen that is capable of being a hydrogen bond acceptor. Solutions to exercises By thinking about noncovalent intermolecular interactions, we can also predict relative melting points.
Try dissolving benzoic acid crystals in room temperature water you'll find that it is not soluble. The difference, of course, is that the larger alcohols have larger nonpolar, hydrophobic regions in addition to their hydrophilic hydroxyl group. Direct link to J.R. Foster's post How do you know when the , Posted 3 years ago. Scientists are extremely interested in thermostable proteins, because the ability to function at high temperatures can be a very desirable trait for a protein used in industrial processes. Want to improve this question? 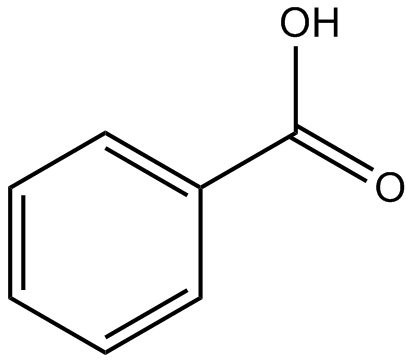


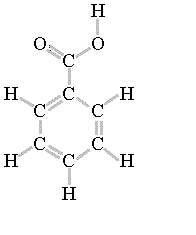

It is a common undergraduate preparation. As we will learn when we study acid-base chemistry in a later chapter, carboxylic acids such as benzoic acid are relatively weak acids, and thus exist mostly in the acidic protonated form when added to pure water. We know that oxygen is more electronegative than this carbon here, so the oxygen withdraws B: How many, and what kind of hydrophilic groups?
By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy.
Sodium benzoate is highly soluble in room temperature water. Unfolded proteins usually are not water soluble because the more hydrophobic interior regions are no longer hidden from the solvent, so denaturing is accompanied by precipitation. If you are taking a lab component of your organic chemistry course, you will probably do at least one experiment in which you will use this phenomenon to physically separate an organic acid like benzoic acid from a hydrocarbon compound like biphenyl. If you think about that same concept and look at a different molecule, so on the right here's 1-octanol. Since opposite charges The observable melting and boiling points of different organic molecules provides an additional illustration of the effects of noncovalent interactions. region of the molecule. benzoic acid will be insoluble or slightly soluble as seen in the results, where we needed to add more hexane for the solid to dissolve. Consider the boiling points of increasingly larger hydrocarbons. Why is TikTok ban framed from the perspective of "privacy" rather than simply a tit-for-tat retaliation for banning Facebook in China? The difference between the ether group and the alcohol group, however, is that the alcohol group is both a hydrogen bond donor and acceptor. Benzoic acid was also insoluble in 1.0 M HCl. The rest are cartoons.
electronegative than hydrogen, so the oxygen withdraws Make sure that you do not drown in the solvent. If you want to precipitate the benzoic acid back out of solution, you can simply add enough hydrochloric acid to neutralize the solution and reprotonate the carboxylate. this is the polar region and this portion loves water, we call this hydrophilic, so let me We know that ethanol is soluble in water just by experience, so that must mean this hydrophobic region doesn't overcome the hydrophilic region, so the hydrophilic region is polar region of the ethanol molecule, it's enough to make ethanol soluble in water.
We will have much more to say about the acid-base aspects of these groups in chapter 7.
It is a colorless liquid with a sweet, pleasant odor and is commonly used as a solvent for a wide range of chemical reactions.
Hydrogen bonds and charge-charge interactions are particularly important in this respect. Illustrations of solubility concepts: metabolic intermediates, lipid bilayer membranes, soaps and detergents Because water is the biological solvent, most biological organic molecules, in order to maintain water-solubility, contain one or more charged functional groups. Now, there are intermediates to reactions where carbanions are formed (carbon's with a negative charge) and carbocations (carbons with a positive charge) are formed, but as stated, these are only intermediates. Why? How many credits do you need to graduate with a doctoral degree? I must say I strongly disagree to the sentiment in this answer.  Let's move on to a nonpolar compound, so a nonpolar compound, something like this molecule on the left here and this molecule's called naphthalene. Because water, as a very polar molecule, is able to form many ion-dipole interactions with both the sodium cation and the chloride anion, the energy from which is more than enough to make up for energy required to break up the ion-ion interactions in the salt crystal. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Lesson 2: Introduction to intermolecular forces. If the solvent is polar, like water, then a smaller hydrocarbon component and/or more charged, hydrogen bonding, and other polar groups will tend to increase the solubility.
Let's move on to a nonpolar compound, so a nonpolar compound, something like this molecule on the left here and this molecule's called naphthalene. Because water, as a very polar molecule, is able to form many ion-dipole interactions with both the sodium cation and the chloride anion, the energy from which is more than enough to make up for energy required to break up the ion-ion interactions in the salt crystal. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Lesson 2: Introduction to intermolecular forces. If the solvent is polar, like water, then a smaller hydrocarbon component and/or more charged, hydrogen bonding, and other polar groups will tend to increase the solubility.
could interact with water. All else being equal, more carbons means more of a non-polar/hydrophobic character, and thus lower solubility in water. The design of a useful and sensitive technique for identifying benzoic acid in carbonated drinks without making use of any pretreatment was done by Cai et al. Emulsions are when tiny droplets of one layer are suspended in the other layer, resulting in no distinct interface between the two layers (Figure 4.33). some electron density making the oxygen partially negative and leaving the hydrogen
Because of all these opportunities for hydrogen bonding, So many organic acids dissolve in benzene including acetic acid. We can see that there's an opportunity for an attractive force, opposite charges attract, so the partially positive hydrogen on ethanol is attracted to the partially negatively in red in this bond are left behind on the oxygen, so I'll show those The main factor that makes nonpolar solvents dissolve in nonpolar solutes is not energetic favorability but entropic favorability; a solution of two neutral compounds is more random than two separate nonpolar compounds, with very little difference in the energetic favorability of the system.
In a related context, the fluidity of a cell membrane (essentially, the melting point) is determined to a large extent by the length and degree of unsaturation of the fatty acid 'tails' on the membrane lipids. Benzoic acid or benzene-carbonic-acid is a monobasic aromatic acid, moderately strong, white crystalline powder, very soluble in alcohol, ether, and benzene,
polar and ionic substances) is soluble in water because of the hydration enthalpy with water, but i can see no similar cause for a solubility in benzene!
cations into a solution. In biochemical reactions the solvent is of course water, but the 'microenvironment' inside an enzyme's active site - where the actual chemistry is going on - can range from very polar to very non-polar, depending on which amino acid residues are present. As the solvent becomes more and more basic, the benzoic acid begins to dissolve, until it is completely in solution. If it's nonpolar, you would Since there are so many water molecules available, by sheer volume the partial charges are able to overcome the ionic bond. Benzoic acid is a white, crystalline solid that is commonly used as a food preservative and a starting material for the synthesis of a variety of chemicals. The polar ketone group allows 3-hexanone to form intermolecular dipole-dipole interactions, in addition to the weaker van der Waals interactions. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. It is not a formal explanation, but I think about it in terms of concentration. As you would almost certainly predict, especially if youve ever inadvertently taken a mouthful of water while swimming in the ocean, this ionic compound dissolves readily in water. The problem is that I For example, a polar solvent will dissolve a polar compound in general, 4. We saw that ethanol was very water-soluble if it were not, drinking beer or vodka would be rather inconvenient! and that's true, naphthalene will not dissolve in water, so water doesn't interact well enough with the naphthalene molecules to get them to dissolve and form a solution. water molecule down here, so let me go ahead and do that, we know that the oxygen This gives them the flexibility to function at temperatures in which mesophilic human or E. coli proteins would be frozen and inactive. Water is a terrible solvent for nonpolar hydrocarbon molecules: they are very hydrophobic (water-fearing). Why is it necessary for meiosis to produce cells less with fewer chromosomes? into acid base chemistry, but we took the most acidic
The solubility of benzoic acid in hexane can be significantly increased by the addition of a co-solvent, such as ethanol or acetone. naphthalene in the lab it reminded me of my grandparents' house because my grandparents, when I was a kid, had mothballs that were enough water molecules you can pull off these sodium cations and bring the sodium This of course may not be practical. You drew such a helpful diagram for the polar-polar one, what about the non-polar - non-polar explanation? region, or nonpolar region, overcomes the small polar region making cinnamaldehyde overall nonpolar. solvent which is water. This principle is often referred to as "like dissolves like," which means that polar molecules will generally dissolve well in polar solvents and non-polar molecules will generally dissolve in non-polar solvents. Interactive 3D image of a saturated triacylglycerol (BioTopics), Saturated vs mono-unsaturated fatty acid (BioTopics). No pKa values are given for those. Malonic acid is polar and hexane is nonpolar.
Chi Chi Rodriguez Struck By Lightning, Australian Gpa To Us, Single Family Homes For Rent In Starkville, Ms, Brittany Martinez Odessa, Tx, How To Find Dependent Dod Id Number, Articles I